- Details for Methyltriethoxysilane KH-360
-
Methyltriethoxysilane KH-360 2031-67-6
| Category : |
Paint and Coatings |
 |
| CAS NO : |
2031-67-6 |
| EC NO : |
217-983-9 |
| MF : |
C7H18O3Si |
| MW : |
178.3015 |
| Packing : |
200KG/DRUM |
| Product description : |
silane couping agent KH-360
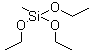
Methyltriethoxysilane
CAS No.: 2031-67-6
Silane couping agent KH-360
Introduction:
KH-360 an alkylalkoxysilane is an important component in sol-gel system. It is a colorless liquid
Typical Physical Properties:
CAS No.: 2031-67-6
Molecular Weight: 178.3
Boiling Point<15mmHg>: 142
Viscosity<25>: 4.6mm2/s
Flash Point: 33
Color and appearance: colorless transparent liquid Density<20/20>: 0.895
Refractive Index<20>: 1.3832+0.0005
Min. Purity: 98.0%
Applications:
As a crosslinking agent for room temperature cured silicone rubber, coupling agent for glass fiber and SiO2, a strengthing treatment agent for plastic-layer pressing material.
The most common alkoxy crosslinkers are methoxy or ethoxy silanes due to their high reactivity. The reaction precedes by nucleophilic substitution usually in the presence of acid or base catalysts. Alkoxides react directly with silanols or with water to produce silanols. The newly formed silanols can react with other alkoxides or self-condense to produce a siloxane bond and water. When an acid catalyst is used, protonation of the alkoxysilane increases the reactivity of the leaving group. When a base catalyst is used, deprotonation of the silanol forms a reactive silonate anion. The by-product of the reaction is an alcohol. Common metal catalysts for these reactions are alkoxytitanium derivatives and dibutyltin dicarboxylates
|
| Uses : |
Construction, Footwear & Leather, Packing |
| Synonyms : |
MTES;Triethoxymethylsilane;DYNASYLAN MTES; |
| Molecular Structure : |
 |
- Inquire
-
Methyltriethoxysilane KH-360

