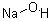| Product description : |
Sodium hydroxide
English alias: Caustic Soda Flakes, Sodium Hydrate、Caustic Soda、Lye
Molecular formula: NaOH
CAS No.: 1310-73-2
EINECS: 215-185-5
Molecular weight: 40.00
Appearance: White crystalline powder
Solubility (mg/L): Easily soluble in water, ethanol, glycerol, insoluble in acetone, ether
Relative density(water=1): 2.13g/cm3
Melting point: 318.4 ℃
Chemical property: Sodium hydroxide is corrosive to fibers, skin, glass, ceramics, etc. It gives off heat when dissolved or diluted in concentrated solution; neutralization reaction with inorganic acid can also generate a lot of heat to produce corresponding salts; reaction with metal aluminum and zinc, non-metal boron and silicon, etc. gives off hydrogen; disproportionation reaction with halogens such as chlorine, bromine and iodine. It can precipitate metal ions from aqueous solution to become hydroxide; it can make saponification reaction of grease to produce sodium salt and alcohol of corresponding organic acid, and thus remove grease on fabric.
Uses: Sodium hydroxide is strongly alkaline and extremely corrosive, and can be used as acid neutralizer, matching masking agent, precipitating agent, precipitation masking agent, color developer, saponification agent, peeling agent, detergent, etc.
Sodium hydroxide is mainly used in the production of paper, cellulose pulp and soap, synthetic detergent, synthetic fatty acid production and the refining of animal and vegetable oils and fats.
Textile printing and dyeing industry used as cotton desizing agent, cooking agent and mercerizing agent.
The chemical industry is used to produce borax, sodium cyanide, formic acid, oxalic acid, phenol, etc.
The petroleum industry is used to refine petroleum products and used in oil field drilling mud.
It is also used in the production of alumina, surface treatment of zinc metal and copper metal, as well as in glass, enamel, tanning, medicine, dyes and pesticides.
Food grade products are used in the food industry as acid neutralizer, as peeling agent for citrus, peaches, etc., and as detergent for empty bottles, empty cans and other containers, as well as decolorizer and deodorizer.
When sodium hydroxide is used as basic reagent, it can be used as neutralizing agent, matching masking agent, precipitating agent, precipitation masking agent, absorbent for small amount of carbon dioxide and water, color developer for determination of ketosteroids by thin layer analysis, etc.
It is widely used in the manufacture of various sodium salts, soap, pulp, finishing cotton fabrics, silk, viscose fibers, regeneration of rubber products, metal cleaning, electroplating, bleaching, etc.
In cosmetic creams, sodium hydroxide and stearic acid saponification as an emulsifier, used to make snow cream, shampoo, etc.
Precautions for storage: Sodium hydroxide should be stored in a shady, dry and well-ventilated storehouse. It should be kept away from fire and heat source. The temperature of the warehouse should not exceed 35℃ and the relative humidity should not exceed 80%.
The package must be sealed, do not get wet. Should be stored separately from flammable (combustible) materials, acids, etc., do not mix storage. The storage area should be equipped with suitable materials to shelter leaks. |