- Search Product
-
-
- Region :China/Zhejiang
- Tel : +86-571-88025872
- Fax : +86-571-88025871
- Email :sales@hzoceanchem.com
- URL :www.hzoceanchem.com
- Add :Room 902,ZEC Development Building, No. 258 Baishi Road, Gongshu District,Hangzhou, Zhejiang,China
- Details for Aluminium Chloride
-
Aluminium Chloride 7446-70-0
Category : Intermediates

CAS NO : 7446-70-0 EC NO : 231-208-1 MF : AlCl3 MW : 133.3422 Specification : AlCl3 ≥99.999% Packing : 50g, 100g/ bottle Product description : Product Name:High purity Aluminium Chloride
Synonym: Aluminum chloride anhydrous
CAS NO: 7446-70-0
Molecular Formula: AlCl3
Molecular Weight:: 133.34
Properties:
Appearance:White granule or powder with strong hydrochloric acid.Industrial products are pale yellow.
boiling point(℃):182.7
melting point(℃):190~194
stability : Soluble in water, ethanol, chloroform, carbon tetrachloride and microbe soluble in benzene
Specification:
AppearanceWhite powder
AlCl3≥99.999%
Cd≤1ppm
Cr≤1ppm
Fe≤1ppm
Pb≤1ppm
Mn≤1ppm
Ni≤1ppm
Mg≤1ppm
Ba≤1ppm
Use: High strength steel has been coated electrochemically by Al in an acidic ionic liquid composed of 1-ethyl -3- methylimidazolium chloride and AlCl3.[1] AlCl3 may be used to form Al-Cd alloy.[2] Anhydrous alumunium chloride is a catalyst in organic chemistry especially for Friedel-Crafts reactions. It may also be used as a nucleation agent in the production of TiO2 pigment by the chloride process.
Packing : 50g, 100g/ bottle
Uses : High strength steel has been coated electrochemically by Al in an acidic ionic liquid composed of 1-ethyl -3- methylimidazolium chloride and AlCl3.[1] AlCl3 may be used to form Al-Cd alloy.[2] Anhydrous alumunium chloride is a catalyst in organic chemistr Synonyms : Aluminum trichloride anhydrous;Aluminum chloride;Aluminum standard concentrate 10.00 g Al;Aluminium chloride anhydrous;Aluminium trichloride;ALUMINIUM CHLORIDE HEXAHYDRATE;Aluminium chloride;Anhydrous aluminum chloride;trichloroaluminum hexahydrate;Aluminum chloride,anhydrous; Molecular Structure : 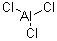
- Inquire
-
Aluminium Chloride