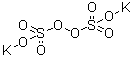| Product description : |
InChI: InChI=1/2K.H2O8S2/c;;1-7-10(5,6)8-9(2,3)4/h;;1H,(H,2,3,4)/q2*+1;/p-2
Potassium persulfate Chemical Properties
mp 1067 °C
bp 1689 °C
density 2.47
vapor density 9.3 (vs air)
solubility H2O: 0.5 M at 20 °C, clear, colorless
Water Solubility 5 g/100 mL (20 ºC)
Merck 14,7656
Stability: Stable. Strong oxidizer. Incompatible with strong reducing agents, organic materials, combustible materials.
CAS DataBase Reference 7727-21-1(CAS DataBase Reference)
EPA Substance Registry System Peroxydisulfuric acid ([(HO)S(O)2]2O2), dipotassium salt(7727-21-1)
Safety Information
Hazard Codes O,Xn
Risk Statements 8-22-36/37/38-42/43
Safety Statements 22-24-26-37
RIDADR UN 1492 5.1/PG 3
WGK Germany 1
RTECS TT5900000
HazardClass 5.1
PackingGroup III
Hazardous Substances Data 7727-21-1(Hazardous Substances Data)
MSDS Information
Provider Language
SigmaAldrich English
ACROS English
ALFA English
Potassium persulfate Usage And Synthesis
Chemical Properties colourless odourless crystals or white powder
General Description A white crystalline solid. Specific gravity 2.477. Decomposes below 100°C.
Air & Water Reactions Water soluble. Slowly decomposed by water. The salt rapidly liberates oxygen when heated, and especially so when wet.
Reactivity Profile Potassium persulfate is an oxidizing agent. Noncombustible but accelerates the burning of combustible material. Potassium persulfate plus a little potassium hydroxide and water released sufficient heat and oxygen to ignite a polythene (polyethylene) liner in a container. [MCA Case History 1155. 1955].
Health Hazard Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Potassium persulfate Preparation Products And Raw materials
Preparation Products Hydrogen peroxide-->Poly(acrylamide)-->8-ACETYL-6-HYDROXY-7-METHOXY-CHROMEN-2-ONE-->FRAXIDIN-->sheeting sensitized glue-->diagnosis latex microsphors combining anti fetoprotein-->self crosslinked type polyacrylate salt super absorbent-->modified acrylic resin emulsion J-->oil-proof, water-proof finishing agent-->3,4-Dinitrobenzoic acid-->rubber latex BA-->Cu(II) imprinted chelating resin-->an improved PVA hydrogel as artificial vitreous body-->brubber latex 202BA-->modified filling agent SBR-->acramoll W paste-->warp dressing agent MVAc-->2,5-DINITROBENZOIC ACID-->rubber latex 109BA-->high-hydroscopic resin (3)-->polymer flocculant-->flocculant TS-614-->Isopropylxanthic disulfide-->filtrate reducer PAC type
Raw materials Sulfuric acid -->Ammonium persulfate-->Ammonium sulfate-->Potassium sulfate |