Chinese VersionChina Suppliers > Hubei new DE sheng material science and technology co., LTD. > What determines the pH value of the buffer solution Bicine?
- Search Product
-
-
- Region :China/Hubei
- Tel : +86-18971041571
- Fax :
- Email :vickyzhao@whdschem.com
- URL :
- Add :Guanggu United Science and Technology City C8, Ezhou City, Hubei Province
- Details for What determines the pH value of the buffer solution Bicine?
-
What determines the pH value of the buffer solution Bicine?
Category : Other Chemicals

CAS NO : 1185-53-1 EC NO : 214-684-5 MF : C4H11ClNO3 MW : 156.5886 Specification : White Powder Packing : 500g/bottle Product description : Biological buffer is usually a widely used auxiliary reagent in biochemical testing and biological experiments, such as Bicine. Bicine buffer has the characteristics of maintaining stable pH value of the system, ensuring reaction progress, promoting enzyme activity, and stabilizing protein nucleic acid structure. One of the key functions is to buffer the changes in the acidity and alkalinity of the system. At this time, the pH value of Bicine buffer solution becomes particularly important, but sometimes it may be affected by some factors, so it is necessary to pay special attention to what determines its pH value? 1、 The pH value is determined by pKa The pH value of Bicine buffer solution is determined by the pKa and buffering ratio of the conjugated acid-base pairs that make up the buffer, but pKa plays a decisive role and buffering has a smaller impact on it. Therefore, after preparing a buffer solvent, without determining which buffer to use, it is necessary to first search for the buffer within the corresponding buffer range based on the required pH value. The closer the pKa of the buffer to the required pH value, the better. In addition, it is also necessary to pay attention to whether the buffer has adverse reactions to the system reaction. If multiple buffer pH values meet the requirements, a buffer solution with good solubility, non-toxic, harmless, strong stability, and easy storage can be selected. 2、 The pH value is determined by adjusting the buffer ratio If a buffer is determined, the change in its pH value may sometimes be affected by the adjustment of the buffer ratio. For example, an increase in the pH value of Bicine will increase the proportion of sodium hydroxide, while a decrease in pH will increase the proportion of Bicine. 3、 The pH value is determined by the buffer concentration, ion strength, and osmotic pressure The higher the concentration of the buffer solution, the better its buffering effect and stronger buffering capacity. However, it should be noted that the higher the concentration, the better. When determining the preparation of the buffer solution, the pH value will also be affected by the total concentration, as well as the influence of ion strength and osmotic pressure on the reaction system. The above three points are the key factors determining the pH range of Bicine buffer solution, and more attention should be paid during use. Desheng Biochemical is a professional manufacturer of biological buffer solutions, such as commonly used Tris, Bicine, MOPS, CAPS, HEPES, etc., which are produced to meet most biological experimental needs. The company also has a large amount of stock, which can be shipped on the same day according to customer needs. The quality is also highly praised by customers and sold overseas. If you have any needs for biological buffering agents, please click on the website to inquire about the details! Uses : Biological buffer Synonyms : Tris(hydroxymethyl)aminoethane hydrochloride;Tris(hydroxymethyl)aminomethane hydrochloride;2-AMINO-2-(HYDROXYMETHYL)-1,3-PROPANEDIOL, HYDROCHLORIDE;SPECTRIS(R) HYDROCHLORIDE;SPECTRIS(TM) HYDROCHLORIDE;MOLE-READY-TRIS;1,3-Propanediol,2-amino-2-(hydroxymethyl)-,hydrochloride;3-propanediol,2-amino-2-(hydroxymethyl)-hydrochloride;trizmahydrochloride(trishydrochloride);2-amino-2-(hydroxymethyl)propane-1,3-diol hydrochloride (1:1);1,3-propanediol, 2-amino-2-(hydroxymethyl)-, chloride;TRIS·HCL;TRIZMA Hydrochloride;Tris Hydrochloride;2-Amino-2-(hydroxymethyl)-1,3-propanediol hydrochloride;Tris.HCl;TRIS-HCL;Tris(hydroxymethyl)amino methane Hydrochloride; Molecular Structure : 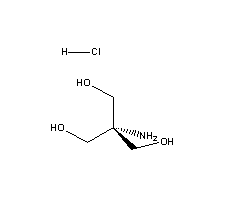
- more>>Other Products
-
- • Biological buffer 3- [N, N-di (hydroxyethyl) amino] -2-hydroxypropanesulfonic acid DIPSO
- • Luminol Sodium Salt
- • 4-Aminophthalhydrazide
- • acridinium ester DMAE-NHS
- • acridinium ester NSP-DMAE-NHS
- • Acridine hydrochloride NSP-SA
- • Acridine hydrochloride NSP-SA-NHS
- • NSP-SA-ADH
- • acridinium ester ME-DMAE-NHS TOOS; 3-(N-Ethyl-3-Methylanilino)-2-Hydroxypropanesulfonic Acid Sodium Salt
- • TOPS; Sodium 3-(N-Ethyl-3-Methylanilino)Propanesulfonate; N-Ethyl-N-Sulfopropyl-M-Toluidine Sodium Salt
- • ADOS Sodium 3-(Ethyl(3-Methoxyphenyl)Amino)-2-Hydroxypropane-1-Sulfonate Dihydrate
- • ADPS N-Ethyl-N-(3-Sulfopropyl)-3-Methoxyaniline Sodium Salt
- • ALPS N-Ethyl-N-(3-Sulfopropyl)Aniline Sodium Salt; Sodium 3-(Ethyl(Phenyl)Amino)Propane-1-Sulfonate; Sodium
- • DAOS; Sodium 3-((3,5-Dimethoxyphenyl)(Ethyl)Amino)-2-Hydroxypropane-1-Sulfonate
- • HDAOS; N-(2-Hydroxy-3-Sulfopropyl)-3,5-Dimethoxyaniline Sodium Salt
- • MADB N,N-Bis(4-Sulfobutyl)-3,5-Dimethylaniline Disodium Salt
- • MAOS N-Ethyl-N-(2-Hydroxy-3-Sulfopropyl)-3,5-Dimethylaniline Sodium Salt Monohydrate
- • DAB 3,3',4,4'-Biphenyltetramine Tetrahydrochloride