Chinese VersionChina Suppliers > Hubei new DE sheng material science and technology co., LTD. > What factors affect the four basic elements of the carbomer 980
- Search Product
-
- Details for What factors affect the four basic elements of the carbomer 980
-
What factors affect the four basic elements of the carbomer 980
Category : Other Chemicals/Others

CAS NO : 54182-57-9 EC NO : 202-415-4 MF : (C3H4O2)n MW : 72.06 Specification : White Powder Packing : 20kg/cardboard box Product description : To better produce and use carbomer 980, we first need to understand its four basic elements: neutralization, viscosity, pH value, and turbidity. So controlling these factors is crucial. Neutralization and viscosity changes of carbomer 980 by different neutralizing bases In the neutralization process, with the addition of alkali, the pH value changes and the viscosity of gel also changes. When arginine, PC-2000, MD-100 and TEA are used as neutralizers, the viscosity of gel shows an overall upward trend with the increase of alkali, and there is only a small range of viscosity fluctuations at high pH values; When KOH is used as neutralizer, before reaching the neutralization point, the viscosity of gel gradually increases with the increase of alkali. If the amount of alkali continues to increase, the pH value of the system will exceed the neutralization point, and the viscosity of gel system will decrease; When NaOH is used as neutralizer, the viscosity of gel is relatively stable, and the gel can reach a relatively high viscosity at the initial stage of neutralization. It can be seen that NaOH in neutralizer has a strong thickening ability zui for Carbomer 980, and the viscosity of gel is less affected by the pH value of the system zui. Neutralization and viscosity changes of carbomer 980 at different concentrations The concentration of carbomer is different, and the initial pH value before neutralization is also different. The higher the concentration, the relatively lower the initial pH value. In the whole neutralization process, with the addition of alkali, the overall change trend of pH value of Carbomer 980 gel with different concentrations is consistent. The lower the concentration of Carbomer, the faster it reaches the neutralization point. The viscosity of gel is positively correlated with the concentration of carbomer. The higher the concentration, the higher the viscosity of gel. The viscosity of gel at the same concentration basically maintains the same level during neutralization. The influence of different electrolyte ions on the viscosity and pH value of Carbomer 980 Carbomer 980 gel is sensitive to electrolyte ions. The existence of electrolyte makes the viscosity of Carbomer 980 gel drop sharply, and the influence of divalent ions is greater than that of monovalent ions. Ions cause the viscosity of the Carbomer gel to decrease, mainly because it reduces the mutual repulsion force of the same charges on the main chain of the polymer, thus reducing the actual effective concentration of Carbomer in the system. Different ions have different effects on the viscosity of Carbomer gel, which is mainly related to the total charge number of ions in the solution. The univalent ions weaken the mutual repulsion through shielding charges, leading to the viscosity decline of Carbomer gel. In addition to this effect, the divalent ions will combine with Carbomer to form salts when their concentration is sufficient, thus further affecting the viscosity and transparency of the gel. The influence of different electrolyte ions on the turbidity properties of Carbomer 980 The introduction of electrolyte ions will also affect the transparency of the Carbomer gel system. Before the addition of electrolyte ions, the Carbomer 980 gel has a colorless and transparent appearance. With the increase of electrolyte ion concentration, the transparency of the gel system has changed, which is reflected in the increase of turbidity. The appearance of the gel system is translucent to opaque. Uses : Biological buffer Synonyms : poly(1-carboxyethylene);Poly(acrylic acid);Acrylic Acid glacial;Fluorosulfuric acid;2-Propenoic acid, homopolymer;Acrylic polymer resins;Polyacrylic Acid;Propenoic acid, homopolymer;Propenoic acid polymer;Propenoic acid, polymers, homopolymer;Carbopol;Carboxypoly-methylene;PAA;Carbomer 910;acrylicacid,poly;Carbomer; Molecular Structure : 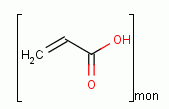
- more>>Other Products
-
- • Biological buffer 3- [N, N-di (hydroxyethyl) amino] -2-hydroxypropanesulfonic acid DIPSO
- • Luminol Sodium Salt
- • 4-Aminophthalhydrazide
- • acridinium ester DMAE-NHS
- • acridinium ester NSP-DMAE-NHS
- • Acridine hydrochloride NSP-SA
- • Acridine hydrochloride NSP-SA-NHS
- • NSP-SA-ADH
- • acridinium ester ME-DMAE-NHS TOOS; 3-(N-Ethyl-3-Methylanilino)-2-Hydroxypropanesulfonic Acid Sodium Salt
- • TOPS; Sodium 3-(N-Ethyl-3-Methylanilino)Propanesulfonate; N-Ethyl-N-Sulfopropyl-M-Toluidine Sodium Salt
- • ADOS Sodium 3-(Ethyl(3-Methoxyphenyl)Amino)-2-Hydroxypropane-1-Sulfonate Dihydrate
- • ADPS N-Ethyl-N-(3-Sulfopropyl)-3-Methoxyaniline Sodium Salt
- • ALPS N-Ethyl-N-(3-Sulfopropyl)Aniline Sodium Salt; Sodium 3-(Ethyl(Phenyl)Amino)Propane-1-Sulfonate; Sodium
- • DAOS; Sodium 3-((3,5-Dimethoxyphenyl)(Ethyl)Amino)-2-Hydroxypropane-1-Sulfonate
- • HDAOS; N-(2-Hydroxy-3-Sulfopropyl)-3,5-Dimethoxyaniline Sodium Salt
- • MADB N,N-Bis(4-Sulfobutyl)-3,5-Dimethylaniline Disodium Salt
- • MAOS N-Ethyl-N-(2-Hydroxy-3-Sulfopropyl)-3,5-Dimethylaniline Sodium Salt Monohydrate
- • DAB 3,3',4,4'-Biphenyltetramine Tetrahydrochloride