Chinese VersionChina Suppliers > Hubei new DE sheng material science and technology co., LTD. > Application of EDTA potassium anticoagulant in biochemical experiments
- Search Product
-
-
- Region :China/Hubei
- Tel : +86-18971041571
- Fax :
- Email :vickyzhao@whdschem.com
- URL :
- Add :Guanggu United Science and Technology City C8, Ezhou City, Hubei Province
- Details for Application of EDTA potassium anticoagulant in biochemical experiments
-
Application of EDTA potassium anticoagulant in biochemical experiments 25102-12-9
Category : Other Chemicals/Others

CAS NO : 25102-12-9 EC NO : MF : C10H12K2N2O8 MW : 366.4086 Specification : White Powder Packing : 500g/bottle Product description : EDTA dipotassium is a white crystalline powder with the molecular formula C10H14N2O8 and a molecular weight of 404.6. It is soluble in water but insoluble in ethanol and other organic solvents. Its chemical stability is good and it has excellent chelating properties. The complex formed by EDTA potassium has high stability for many metal ions, making it a strong and effective chelating agent. It is not easily decomposed under normal conditions, but extreme conditions such as high temperature, strong acid or strong alkali may affect its stability. EDTA dipotassium can form stable complexes with calcium ions in the blood, thereby preventing blood coagulation. Its anticoagulant effect mainly depends on the formation of soluble complexes with calcium ions, reducing the concentration of calcium ions in the blood, and thereby inhibiting the activation of prothrombin and the activity of coagulation factors. In addition, EDTA dipotassium can also chelate other metal ions in the blood, such as iron, copper, etc., stabilizing blood components and protecting blood cells from damage. application area 1. Hematology: In hematology, EDTA dipotassium is widely used as an anticoagulant in blood collection tubes to prevent calcium ions from participating in the coagulation process, thereby maintaining the original state of the blood. Test tubes containing this anticoagulant are commonly used for blood routine tests, blood type identification, and other tests. 2. Biochemistry: The chelating properties of EDTA make it an ideal reagent for separating and detecting metal ions. When analyzing the metal content in biological samples, the use of EDTA can exclude other interfering substances. 3. Molecular biology: In molecular biology experiments, the application of EDTA dipotassium is mainly reflected in the extraction process of DNA and RNA. Due to its chelating effect on metal ions, EDTA can prevent the activity of nucleases, thereby protecting the integrity of nucleic acids. Uses : Blood collection additive Synonyms : EDTA-2K•2H20;2K;EDTA, Dipotassium Salt;Ethylenediaminetetraacetic acid, dipotassium salt;Ethylenediaminetetraacetic acid, dipotassium salt dihydrate;Ethylenediamine-N,N,N',N'-tetraacetic acid, dipotassium salt, dihydrate;Ethylenediaminetetraacetic acid,dipotassium salt dihydrate;EDTA-2K·2H20;acetate, 2,2',2'',2'''-(1,2-ethanediyldinitrilo)tetrakis-, potassium salt (1:2);Ethylenediaminetetraacetic acid dipotassium salt dihydrate;Ethylenedinitrilotetraacetic acid dipotassium salt dihydrate; Molecular Structure : 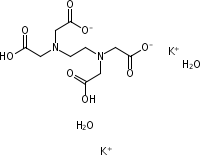
- Inquire
-
Application of EDTA potassium anticoagulant in biochemical experiments