Chinese VersionChina Suppliers > Hubei new DE sheng material science and technology co., LTD. > Disodium ethylenediaminetetraacetic acid/EDTA N2
- Search Product
-
-
- Region :China/Hubei
- Tel : +86-18971041571
- Fax :
- Email :vickyzhao@whdschem.com
- URL :
- Add :Guanggu United Science and Technology City C8, Ezhou City, Hubei Province
- Details for Disodium ethylenediaminetetraacetic acid/EDTA N2
-
Disodium ethylenediaminetetraacetic acid/EDTA N2 6381-92-6
Category : Others

CAS NO : 6381-92-6 EC NO : 205-358-3 MF : C10H20N2Na2O10 MW : 374.2516 Specification : White Powder Packing : 500g/bottle Product description : EDTA disodium can coordinate with most metal ions to form a very stable cyclic chelate. Due to its stoichiometric coefficient of chemical reaction with metal ions generally being 1:1, it is more convenient to calculate, which is also one of the reasons why it is widely used. EDTA disodium salt is an abbreviation for ethylenediaminetetraacetic acid disodium salt. It appears as a white crystalline powder, odorless, non-toxic, low hygroscopicity, soluble in water, and has a saturated solution concentration of about 0.3mol/L at room temperature. It is usually prepared into a standard solution of 0.01-0.1mol/L for titration analysis. In order not to affect the accuracy of EDTA disodium standard solution, we should pay attention to the following points during the configuration process: 1. When weighing samples, attention should be paid to accurate dosage to avoid errors during configuration; 2. The solution used in the analysis experiment should be prepared with pure water, and the container should be washed with pure water at least three times; 3. When shaking well, the bottle mouth should be tightly closed and attention should be paid to the tightness of the bottle stopper to avoid leakage and loss; 3. The prepared solution cannot be placed in a regular glass bottle, as the Ca2+and Mg2+in the bottle will react with EDTA and affect the results. It is best to use a polyethylene bottle or a hard glass bottle; 4. When using the calibration method to prepare EDTA disodium standard solution, attention should be paid to the quality of distilled water. If the water contains Ca2+, Mg2+, Al3+, Fe3+plasma, it will consume titrant or blocking indicator, which will affect the results. Uses : Blood collection additive Synonyms : DISODIUM DIHYDROGEN ETHYLENEDIAMINETETRAACETATE DIHYDRATE;Disodium dihydrogen ethylenediamine tetraacetic dihydrate;Disodium edathamil dihydrate;Disodium edetate dihydrate;Disodium(ethylene dinitrilo)tetraacetic acid dihydrate;Disodium ethylenediaminetetraacetate dihydrate;Cheladrate dihydrate;Chelaplex III;Edathamil disodium dihydrate;Edetate disodium dihydrate;Edetic acid disodium salt dihydrate;EDTA-2Na dihydrate;EDTA, Disodium Salt;Endrate disodium dihydrate;Ethylenediaminetetraacetic acid, disodium salt, dihydrate;ETHYLENEDIAMINE TETRAACETIC ACID dihydrate;Ethylenebis(iminodiacetic acid) disodium salt dihydrate;(Ethylenedinitrilo)-tetraacetic acid disodium salt dihydrate;N,N'-1,2-Ethanediylbis[N-(carboxymethyl)glycine] disodium salt dihydrate;Sequestrene NA 2 dihydrate;Sodium Versenate dihydrate;Tetracemate disodium dihydrate;Titriplex III dihydrate;Versene disodium salt dihydrate;EDTA-2NA 2H2O;sodium 2,2'-{ethane-1,2-diylbis[(carboxymethyl)imino]}diacetate hydrate (2:1:2) (non-preferred name);disodium 2-[2-(bis(carboxymethyl)amino)ethyl-(carboxymethyl)amino]acetic acid dihydrate;Ethylene diamine tetra acetic acid diso-dium salt dihydrate;Disodium EDTA dihydrate;Ethylenediaminetetraacetic disodium salt dihydrate;Ethylenediaminetetraacetic acid disodium salt dihydrate;EDTA 2Na dihydrate; Molecular Structure : 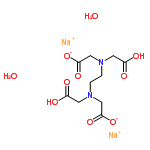
- Inquire
-
Disodium ethylenediaminetetraacetic acid/EDTA N2