Chinese VersionChina Suppliers > Hubei new DE sheng material science and technology co., LTD. > Piperazin-N, N '- bis (2-ethanesulfonic acid) PIPES
- Search Product
-
-
- Region :China/Hubei
- Tel : +86-18971041571
- Fax :
- Email :vickyzhao@whdschem.com
- URL :
- Add :Guanggu United Science and Technology City C8, Ezhou City, Hubei Province
- Details for Piperazin-N, N '- bis (2-ethanesulfonic acid) PIPES
-
Piperazin-N, N '- bis (2-ethanesulfonic acid) PIPES 5625-37-6
Category : Others

CAS NO : 5625-37-6 EC NO : 227-057-6 MF : C8H18N2O6S2 MW : 302.36 Specification : White Powder Packing : 500g/bottle Product description : PIPES, also known as 1-4-piperazine diethyl sulfonic acid in Chinese, is an amphoteric buffer that is insoluble in water but soluble in NaOH aqueous solution. It has good buffering capacity in the pH range of 7.2 to 7.4. PIPES belongs to a type of Good's buffer solution and is widely used in tissue culture, vaccine production, cation efficacy research, bacterial culture, fungal protoplast culture, electron microscopy, and biological material separation technology. The pH buffer range of PIPES is 6.1 to 7.5, insoluble in water, and soluble in NaOH aqueous solution. PIPES is different from buffering agents containing bis (2-hydroxyethyl) amino groups (such as Bis tris, Bicine) and cannot form stable complexes with most metal ions. It is suitable for buffering agents in solution systems containing metal ions. PIPES has two pKa values. 1pKa (6.76 at 25 ° C) is close to physiological pH, making it suitable for cell culture work. Its effective buffering range is 6.1 to 7.5 at 25 ° C. The second pKa value is 2.67, with a buffer range of 1.5-3.5. There are literature records that PIPES can minimize lipid loss when buffering glutaraldehyde histology in plant and animal tissues. The ability of PIPES to bind to divalent ions is negligible. PIPES can be used to purify tubulin by cellulose phosphate chromatography, to purify recombinant GTP binding proteins ARF1 and ARF2 by gel filtration, and to crystallize transketolase from Escherichia coli as a buffer solution. In addition, due to its ability to form free radicals, PIPES is not suitable for application in redox systems. In cation exchange chromatography, low concentration PIPES buffer should be used because PIPES has relatively high ion strength and its pKa value is concentration dependent. Uses : Biological buffer Synonyms : PIPES, Free Acid, ULTROL Grade;2,2-(piperazine-1,4-diyl)bis(ethanesulphonic) acid;PIPES Piperazine-N,N-bis(2-ethanesulfonic acid);PIPES, Molecular Biology Grade Piperazine-N,N-bis(2-ethanesulfonic acid), Molecular Biology Grade;PIPES, Free Acid, MB Grade (1.12030);1,4-Piperazinebis(ethanesulfonic acid);Piperazine-1,4-bis(2-ethanesulfonic acid);Piperazine-N,N'-bis(2-ethanesulfonic acid);Piperazine-1,4-bisethanesulfonic acid;LABOTEST-BB LT00138111;1,4-PIPERAZINEDIETHANESULFONIC ACID;PIPERAZINE-NN'-BIS-2-ETHANESULPHONIC ACID;TIMTEC-BB SBB008973;PIPES; Molecular Structure : 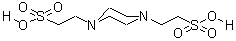
- Inquire
-
Piperazin-N, N '- bis (2-ethanesulfonic acid) PIPES